2. Scientific achievements
2.1. WP1. Microbial interactions in synthetic communities
2.1.1.Involved partners
All Belgian partners (UGent, UCL, KUL, UMons) were involved with WP1. In WP1 robust synthetic communities are developed to serve further for experimentation in WP2 through 4. P1 (UGent, CMET) was the WP leader.
2.1.2.Summary description of the objectives
WP1’s main objective was to use simple but high throughput approaches to test the effect of microbial community composition (richness, evenness, diversity, architecture, species characteristics…) on certain ecological phenomena, such as invasion, horizontal gene transfer, different trophic strategies …
2.1.3.Summary of the scientific activities and results per partner.
P1: UGent (CMET)
Objectives
Task 1.1. Sand filter ecosystems:
- To generate a big library of synthetic communities of sand filter isolates with variable evenness but a fixed 10-species richness (194 synthetic communities) for use by the whole consortium.
- Validate novel diversity measurement technique (Props et al. , in press.) by means of this synthetic communities.
Task 1.2. Soil ecosystems:
- Increase the genome availability of isolates from hydrocarbon-contaminated environments
Scientific activities and results
Task 1.1. Sand filter ecosystems:
Synthetic communities were generated with different evenness but with constant richness (10 species). Synthetic communities were subsequently incubated and transferred five times in fresh LB medium (Miller) at 28°C. Each cycle lasted 48h. The total cell count was assessed by flow cytometry (Accuri C6, BD) in all time points and relative quantification of the different members of the synthetic communities was followed by amplicon sequencing of the 16S rRNA gene (Illumina MiSeq) for two time points: time zero and five.
Similarities between the time zero and time five (with 3 different evenness levels (low, medium and high)) were analyzed by means of multiple factor analyses. The result showed that there was no correlation between the community structure of the two time points but, as expected, high correlation between three different evenness was observed (Figure 2‑1).
Using a least squares means comparison (SAS PROC LSMEANS) it was observed that the evenness changed during the incubation time and the significant effects could be observed in the different time points (tpt), the interaction effect evenness*tpt and the different evenness groups with different indices like the Pielou and Shannon diversity indices (Table 2‑1). Negative correlation was found between some species which could be attributed to a decrease in total species over time.
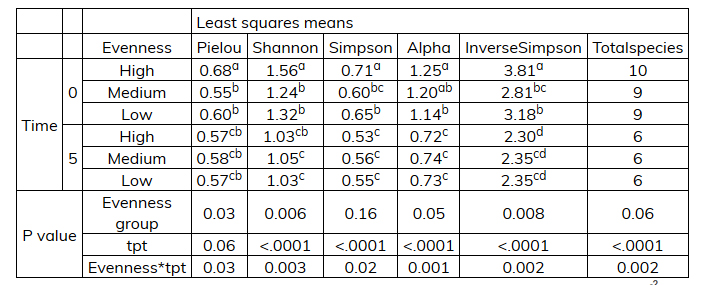
Table 2‑1. Results of least squares means analysis.
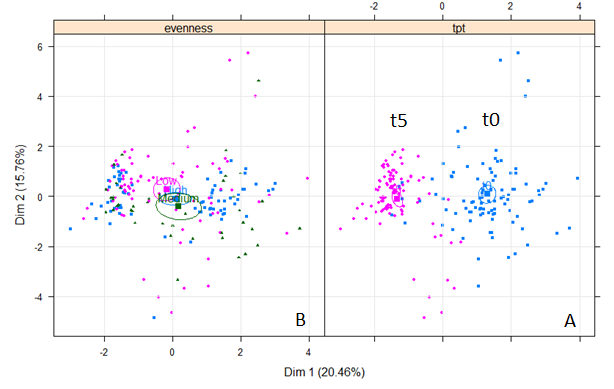
Figure 2‑1. Result of multiple factor analysis. (A) No correlation between two time points. Blue color for time point 1 and Pink color for time point 5. (B) Correlation between three different evenness. Pink color for Low, green color for Medium and blue color for High evenness.
Overall, the community structures converged over time, irrespective of the initial evenness level:
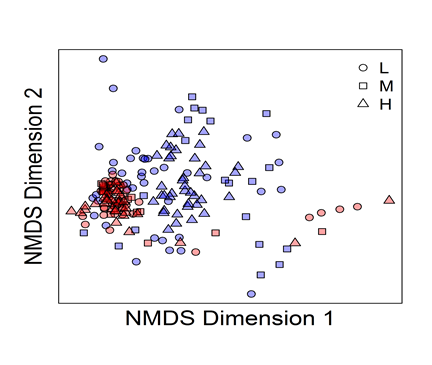
Figure 2‑2. The beta diversity plot showed that the majority of communities evolved toward the same structure. Blue color time 0 and red color time 5.
Using SYBR-green based flow cytometry and a multi-dimensional diversity assessment based upon flow-cytometric scatter (Props et al. , in press.). The community diversity could be assessed for each separate time-point. The result of flow cytometry showed the decreasing trend in total cell counts over time when the (initial) evenness was low (and, to a lesser extent, when evenness was medium). Diversity also decreased over time specially in synthetic communities with high evenness and showed that communities with high evenness were more influenced by prolonged co-cultivation than the other communities with low or medium evenness (Figure 2‑3). Furthermore, we observed that the variability in total count over time was greatly reduced in the case of low and high initial evenness while this was not the case for medium evenness. Exact mechanistic interpretation of the data is currently under investigation.
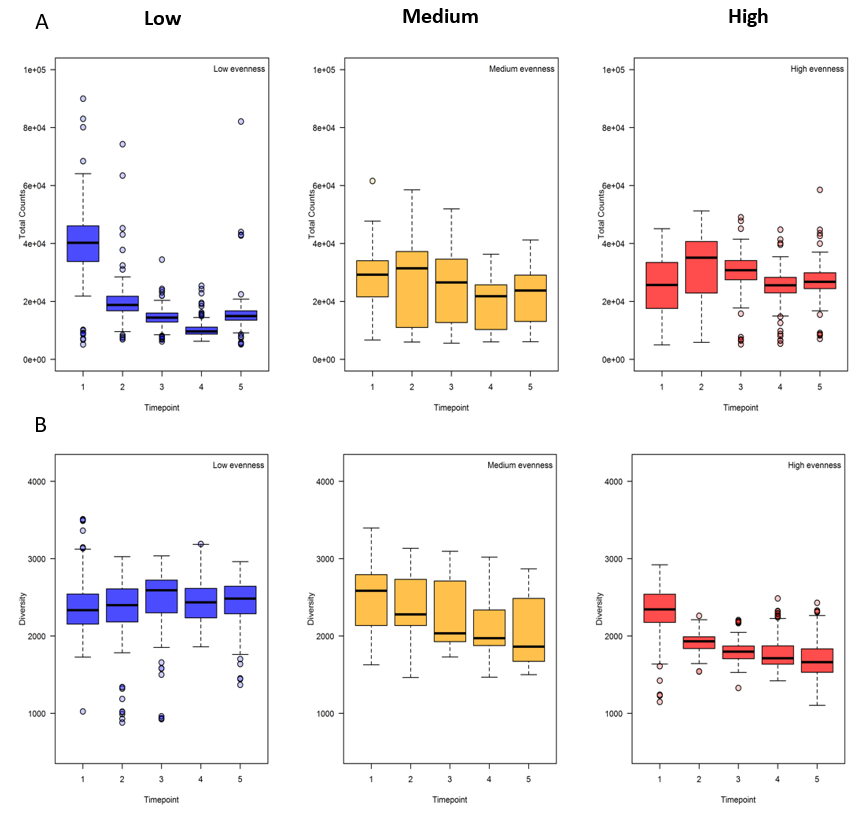
Figure 2‑3. Results of flow-cytometric analysis (A) Changes in total cell counts of synthetic communities with low, medium and high evenness and (B) Diversity over time. Blue color Low, yellow color medium and red color high evenness.
Task 1.2. Soil ecosystems:
- No genomes were available for non-endophytic (root associated diazotrophic) Herbaspirillum , although the genus harbors great potential for species related to biodegradation processes. Hence we aimed at increasing the knowledge of the genomic diversity within the genus Herbaspirillum by extending it with genomes of isolates from sites contaminated with PAH
- The draft genome of Herbaspirillum strain naphatalenivorans RV1432 was sequenced using Illumina MiSeq, and automatically annotated using RAST.
- Comparative genomics with Herbaspirillum lusitanum (99% 16S rRNA identity) showed many unique ORFs and RV1432 was found to be the first strain in the genus described to contain a complete gentisate pathway for degradation of naphthalene. Strain RV1432 also contained evidence to be able to uptake DNA by HGT.
- This fully genetically characterized isolate was subsequently challenged to invade a synthetic community of 21 isolates with enough phylogenetic diversity to allow RV1432 to be picked up by Illumina MiSeq (described in the 2012-2013 report).
- The draft genome of Rhodococcus sp. strain 311R, isolated from a soil contaminated with alkanes and aromatic compounds was sequenced using Illumina MiSeq.
- The draft genome of Aeromonas Strain EERV15, isolated from a sand filter (obtained from KUL) was sequenced using Illumina MiSeq.
Main achievements in respect to initial WP objectives
- The behavior of a synthetic community of 10 isolates was characterized by means of amplicon sequencing and a novel flow cytometric diversity assessment approach and is now available for the network and further experimentation
- Draft genomes of Herbaspirillum strain naphatalenivorans RV1432 and Rhodococcus sp. strain 311R were published. A third genome of Aeromonas sp. Strain EERV15 is in prODration.
-
P1: UGent (KERMIT) – supporting role (to P3)
Objectives
Task 1.1: To investigate how the diversity of the endogenous community affects the invasion of pesticide degrading inoculants in sand filter ecosystems.
Scientific activities and results
- Performed statistical analyses in order to determine the presence and significance of correlations between BAM-degradation performance of MSH1 (as measured by mineralization parameters) and the presence of (combinations of) sand filter isolates
- Performed statistical analyses in order to determine the presence and significance of correlations between BAM-degradation performance of MSH1 (as measured by mineralization parameters) and the survival of MSH1 after two competition phases
- Performed clustering analysis in order to select more specific richness conditions for a more detailed screening of diversity effects
Main achievements in respect to initial WP objectives
Task 1.1:
- The results of the statistical analyses were used to help determine how the diversity (richness) of the endogenous community affected the degradation of BAM by MSH1 in sand filter ecosystems
- The results of the clustering analysis were used in order to design a second set of experiments investigating the effect of the endogenous community diversity on BAM-degradation by MSH1
P2: UCL
Objectives
Task 1.2, Soil ecosystems
In this task polycyclic aromatic hydrocarbons (PAH) biodegradation using synthetic bacterial communities (consisting of Proteobacteria and Acidobacteria, described in previous reports) was investigated.
Task 1.3, Marine Ecosystems
As an objective related to marine ecosystems, dimethylsulfoniopropionate (DMSP) biotransformation and sulfur cycling using bacterial communities constructed with different marine bacterial strains was investigated (see previous reports).
Additional Task: Wastewater ecosystems
As an additional objective related to wastewater ecosystems, treatment of synthetic wastewater at 4 °C using artificial, psychrophilic bacterial communities was investigated.
Scientific activities and results
Task 1.2, Soil ecosystems
- Selection of PAH-degrading bacterial strains (i.e., Sphingomonas strain LB126 (fluorene (FLU)-degrading strain), Novosphingobium sp. strain LH128 (phenanthrene (PHE)- degrading strain), Mycobacterium sp. strain LB501T (anthracene (ANT)- degrading strain) and Mycobacterium sp. strain LB208 (pyrene (PYR)-degrading strain).
- Biodegradation kinetics of individual PAHs using combinatorial biodiversity experiments (full factorial design, from monocultures to tricultures).
- Biodegradation kinetics of a mixture of PAHs (PHE, FLU, ANT) using combinatorial biodiversity experiments (full factorial design, from monocultures to tricultures, Figure 2‑4).
- Analysis of biodiversity effects on productivity (biomass production yield) and functionality (biodegradation) (e.g., additive, tripartite partition of observed yield differences (Fox’s Equation)).
- Improved biodegradation rates and yields of PAH mixtures using synthetic mixed cultures compared to monocultures.
- Whole-genome sequencing of fluorene-degrading Sphingomonas strain LB126 (PacBio sheared gDNA library and PacBio sequencing SMRT cell).
- Design of qPCR assays for quantitation of individual populations
- Bioaugmentation of artificial mixed cultures representative of soil ecosystems using PAH-degrading bacterial cultures (bioaugmentation formulations).
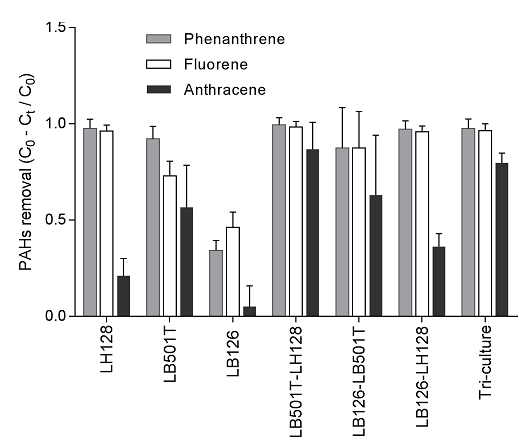
Figure 2‑4. Comparison of PAHs removal (a mixture of fluorene, phenanthrene and anthracene, ca. 50 mg L-1 each) in the presence of mono-cultures, co-cultures and tri-cultures after an incubation time of 12.8 d. Total, initial cell concentrations were set to 2.0 * 107 cell mL-1. Substitutive design (replacement series) was used in which the total, initial cell concentration of the community is maintained constant (i.e., the initial cell concentration for each individual population decreased with an increase of species richness).
Task 1.3, Marine Ecosystems
- Selection and isolation of DMSP-degrading bacterial strains (Halomonas strain HTNK1, Ruegeria sp. strain TM1040, Roseobacter pomeroyi strain DSS-3, Dinoroseobacter shibae strain DFL12, Stappia stellulata (isolate #A), Alteromonas macleodii (isolate #B)).
- Combinatorial biodiversity experiments (coupling between biodiversity, functionality and niche dimensionality (one carbon source to multiple carbon sources, including algal extracts (e.g., from DMSP-producing Emiliania huxleyi bloom)).
- Design of qPCR assays for quantitation of individual populations
- Improved productivity in communities with higher species richness (Biodiversity-function coupling)
Main achievements in respect to initial WP objectives
Task 1.2, Soil ecosystems
PAH biodegradation by synthetic bacterial communities and coupling of biodiversity and multifunctionality (biodegradation of different PAHs and biomass productivity, Figure 2‑5).
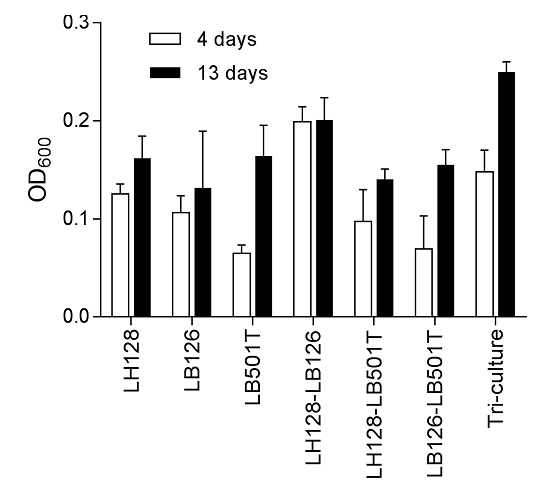
Figure 2‑5. Comparison of OD600 values after 4 days and 13 days of incubation in the presence of a mixture of fluorene, phenanthrene and anthracene, ca. 50 mg L-1 each.
Task 1.3, Marine Ecosystems
DMSP biodegradation by synthetic bacterial communities and coupling of biodiversity and functionality. The net biodiversity effect (NBE) measures the deviation of the mixture yield as compared to the expected value coming from monoculture yields and the relative abundance of species in the mixture. It’s different components are the dominance effect (DE), the trait-dependent and trait-independent complementarity effects (TDC and TIC) as explained in previous reports. When a synthetic consortium was grown on a mixture of carbon sources, the dominance effect strongly increased with a concomitant decrease in TIC and TDC with increasing phylogenetic diversity. Whereas if a medium containing only DMSP as a carbon source leads to dominance of TIC in the NBE (Figure 2‑6).
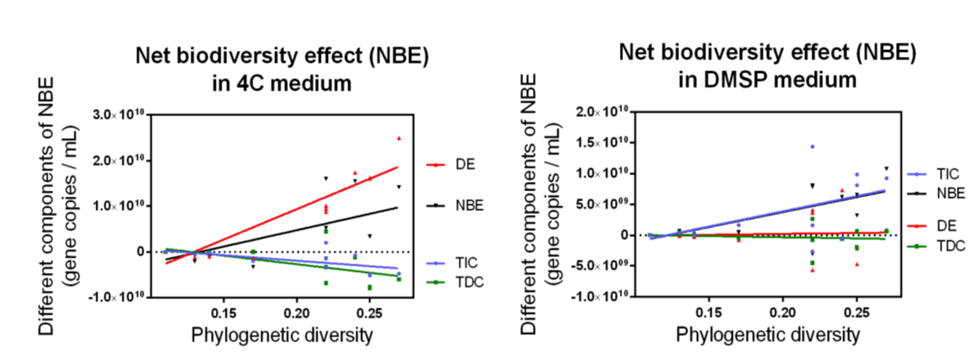
Figure 2‑6. Biodiversity effects (PD) obtained through Fox equation applied on the data resulting from qPCR reactions on the different synthetic communities grown in the presence of 4 different carbon sources. TIC, p= 0.0059; TDC, p = 0.0118; DE, p < 0.0001; NBE, P = 0.007. DE: dominance effect, TDC: trait-dependent complementarity effect, TIC: trait-independent complementarity effect.
Additional task, wastewater ecosystems
- Selection and isolation of psychrophilic strains able to remove COD of synthetic wastewater formulated on the basis of one inhabitant equivalent (Devosia psychrophila DSM 22950, Devosia glacialis DSM 23846, Hymenobacter psychrophilus DSM 22290, Arthrobacter psychrolactophhilus Sp 31.3 (from ULg, Prof. Feller), Brevindumonas (isolate #A), Pseudomonas sp. (isolate #B)).
- Formulation of synthetic wastewater (Inhabitant Equivalent, SPGE, Public Water Management Company, 750 mg COD L-1 (i.e., 135 g COD per capita per day), 55 mg L-1 Ntot and 11 mg L-1 Ptot).
- Enhanced removal of soluble COD in microbial communities with higher species richness
P3: KUL
Objectives
Task 1.1:
- Determine the influence of endogenous diversity on invasion of sand filter ecosystems
- Identify genes involved in “interactive behavior” of Aminobacter s MSH1 with sand filter isolates
Task 1.2:
- Examine how different types of PAH degraders affect each other’s activity when colonizing the same micro-niche
Scientific activities and results
Task 1.1:
- Bacteria endogenous to the sand filter environment (seven different water works providing drinking water from ground and surface water) were isolated using simple isolation procedures, i.e. diluted media and prolonged incubation times. 26 selected pure cultures were characterized as typical inhabitants of oligotrophic environments such as sand filters used in drinking water treatment.
- 13 sand filter isolates (SFI) were used to study the influence of C-source competition and other interspecies interactions on invasion of the BAM degrader Aminobacter MSH1 in high throughput dual and triple species microcosm ecosystems. Results indicated both competitive and cooperative interspecies interactions. Survival of MSH1 and resulting BAM mineralization depended on the competitive behavior and identity of the SFI present.
- The same 13 SFI were used to study the influence of species richness on the invasion of MSH1 in sand filter microcosms consisting of MSH1 combined with different combinations of SFI ranging between richness 1 and 13. With increasing richness, variability of the results decreased and survival of MSH1 decreased, resulting in impaired BAM mineralization (Figure 2‑7 and Figure 2‑8). Deeper data-analysis to unravel how species richness affects invasion in addition to interspecies interactions is on-going.
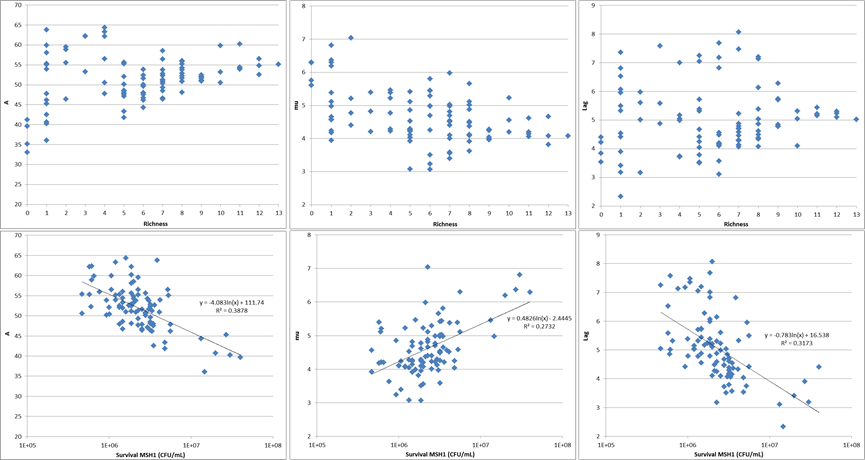
Figure 2‑7. Results of competition experiment based upon covering array design for increasing richness (DIVEXP7) showing the decrease in variability with increasing richness as well as the impairment of the BAM mineralization with decreased MSH1 survival as well as a logarithmic relationship between MSH1 cell number in presence and absence of SFI.
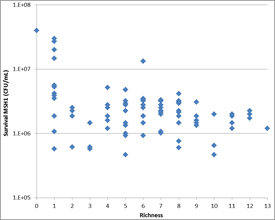
Figure 2‑8. Illustration of reduced MSH1 survival with increased SFI richness
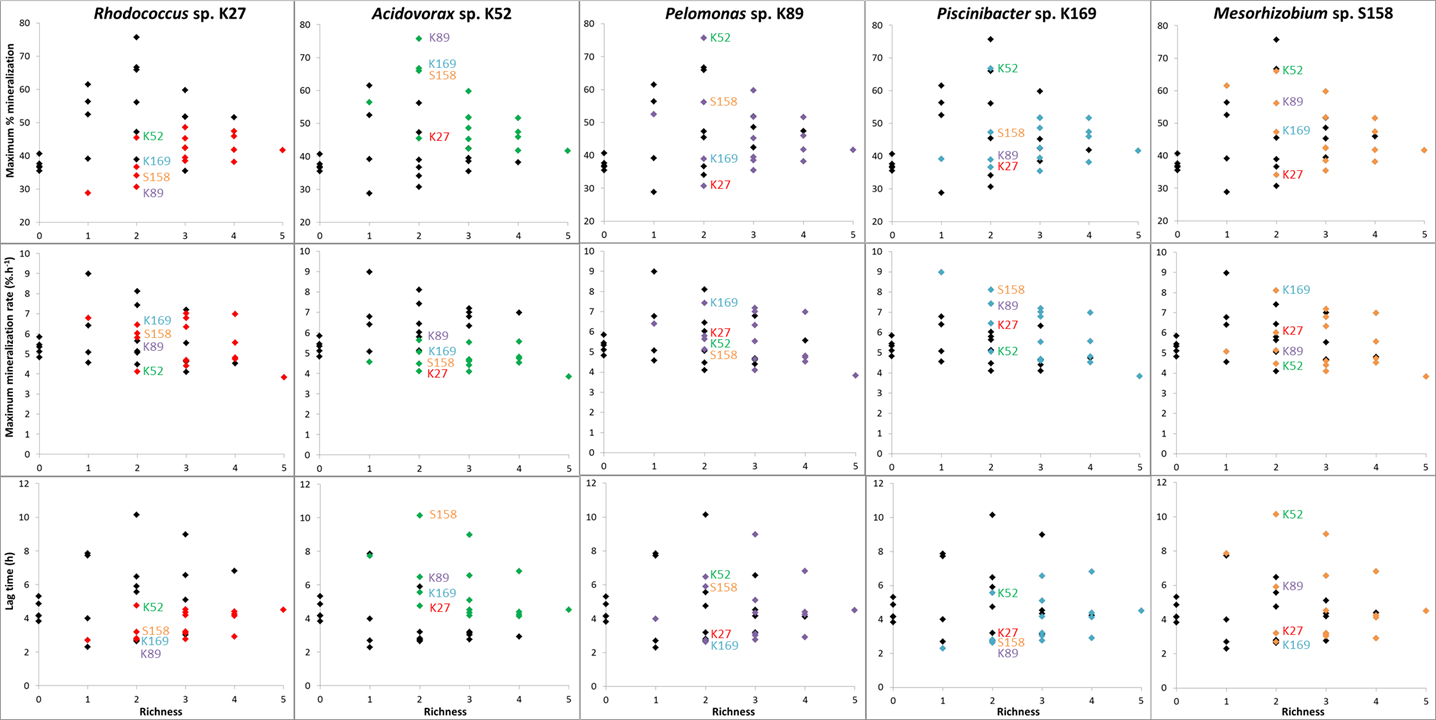
Figure 2‑9. Specific effects of SFI identity on MSH1 survival and modelled parameters of BAM removal curves.
- Invasion of MSH1 and BAM mineralization were evaluated for combinations of MSH1 and 5 selected SFI. Individually combined with MSH1, the selected SFI had diverging effects on invasion of MSH1. To study their collective effect and the competitive behavior among the SFI and MSH1 when richness increases, all possible combinations of MSH1 and SFI were tested, with SFI richness ranging from 1 to 5. Explanations for the interactive behavior of SFI will be sought by studying their genome sequences, which are being assembled at the moment.
- The use of synthetic communities or extracted communities from sand filter samples didn’t render any difference in the outcome of the aforementioned scenarios under similar conditions.
- Different approaches were developed to identify “interactive genes” in Aminobacter MSH1 when in contact with SFI under conditions of oligotrophy. These included Differential Fluorescence Induction (DFI), Transposition INsertion SEquence (TraDIS) and differential RNAseq. Differential proteomics (UMons) was abandoned because of too low biomass. Several problems were encountered including low biomass and genetic instability of the strain MSH1 regarding the BAM catabolic features. These problems were solved but led to substantial delay in reaching the objectives. Sequencing of all the libraries (DFI, TraDIS, RNAseq) will be performed in the coming months. This work nevertheless resulted into a better understanding of the genes involved in BAM degradation in MSH1 (present on two catabolic plasmids) (T’Syen et al., 2015) and the importance of genetic stability of the catabolic genes within this strain to function in sandfilter ecosystems.
Task 1.2:
- Based on previous knowledge, it was examined how different bacterial species able to degrade the same PAH compound (phenanthrene) behave and affect each other’s PAH degrading activity when being together. It was found that the presence of a phenanthrene degrading Pseudomonas putida OUS82 strongly affects the survival and metabolism of a phenanthrene degrading Mycobacterium VM552, probably due to the production of a metabolite from phenanthrene degradation. Differential proteomics (with UMons) indicate that the presence of strain VM552 upregulated expression of phenanthrene degradation in OUS82 (see previous reports).
Main achievements in respect to initial WP objectives
Fundamental knowledge was generated with respect to how the diversity of the endogenous community influences invasion of strain MSH1 of a sand filter community. Besides species richness, species identity plays an important role. Different approaches were developed to identify “interactive genes” in Aminobacter sp. MSH1 when in contact with sand filter isolates under conditions of oligotrophy. Bacterial species degrading the same organic pollutant can affect each other growth and activity.
Deviations from initial WP objectives
- Species richness was assayed instead of evenness, allowing a more straightforward investigation of species identity effects and of specific interactions between MSH1 and SFI.
- Invasive behaviour of MSH1 in sand filter environment was assessed and how this is influenced by the indigenous community and the availability of additional organic carbon for growth
- Delay was encountered in the identification of “interactive genes” in MSH1 and hence in the creation of mutants in those interactive genes. These mutants are as such not available to test their behaviour in subsequent WPs.
P4: UMons
Objectives
Task 1.3, Marine Ecosystems
The main objective of WP1 was to evaluate invasion of microbial communities. For that, the UMons partner developed two model synthetic communities were established in the laboratory, one in a marine and another one in a freshwater environment (see previous reports). The impact of microbial diversity and the role of HGT (horizontal gene transfer) were also studied. A large part of this WP was devoted to design the tools used in the research project: the synthetic microbial communities as such, as well as accurate methods to analyse their composition and function.
Scientific activities and results
Task 1.3, Marine Ecosystems
Two major advances have been made :
1) For (meta)proteomics, we have implemented a State-of-the-Art mass spectrometry (MS) acquisition approach, the SWATH-MS (Sequential Window Acquisition of all THeoretical fragment ions). SWATH-MS was then used to analyse a synthetic community elaborated for marine sediments (a manuscript will be submitted soon on this point). It represents a major improvement in the field of microbial ecology. The marine synthetic community was composed of 9 bacteria: Shewanella baltica OS155, Shewanella frigidimarina ACAM591, Burkholderia glumae LMG2196, Burkholderia xenovorans LB400, Mycobacterium vanbaalenii PYR-1, Methylibium petroleiphilum PM1, Cupriavidus metallidurans CH34, Escherichia coli BL21-DE3 and Pseudomonas putida SP902. These strains were selected based upon differential abundance of different genera with freshwater sediments (as determined by metaproteomics) in North Sea marine sediments at Station 130 (see the 2012-2013 reporting period). These bacteria have been cultured together for one month in SSM8 medium. At day 4 and 28, DNA extractions and qPCR experiments have been conducted in order to assess the composition of the community. At the end of incubation, the community was mainly composed of Pseudomonas putida and Shewanella baltica. At 4 and 28 days as well, hydrophilic proteins were prepared using the bead beater technology and subjected to trypsin digestion. SWATH-MS was then used. In parallel, single bacterial cultures have been prepared for each strain in the same medium and conditions, and hydrophilic proteins have been prepared according to the same protocol. Basic DDA acquisition (Data Dependent Acquisition, i.e. not the SWATH approach) was then used. Using a library composed of 9 strains we prepared a spectral library for 3 of the strains (a spectral library is a library composed of all the possible transitions obtained in one condition for one strain in particular). We then matched this library against the SWATH-MS data of the whole synthetic community. Using this approach, bacteria that were previously invisible became visible: indeed, we could detect 85 proteins from C. metallidurans as well as 76 proteins from E. coli (it was shown before by qPCR analysis that these bacteria were present in very low abundances in the synthetic community). Previous mass spectrometry approaches were not able to detect these bacteria (see previous reporting session). Thanks to the SWATH-MS approach we may suggest that E. coli is no more viable in the community, contrary to C. metallidurans that is totally functional. This conclusion may be drawn because the excreted proteome is visible (little proteins used as sensors, to interact and certainly adapt to the environment; see further details in WP2). The SWATH-MS approach proved very useful to investigate the metabolism of rare bacteria in a complex community. This may be highly relevant as investigations into the metabolism of the rare members of the microbiome have so far been precluded due to the low abundance of these members. Although very promising in its present state, the SWATH-MS approach may still be improved, for instance for the quality and peak quantity of the spectral library. A first manuscript presenting the SWATH-MS approach as a major improvement in the field of environmental microbiology (Beraud et al., in preparation) will be submitted soon. A second manuscript focused on the functionality of the synthetic community will follow.
2) A river synthetic community was also successfully prepared on the basis of the natural community located in the Scarpe river in Férin (Northern France; Gillan et al. 2015). It is composed of 9 strains : Azoarcus communis LMG22127, Burkholderia glumae LMG2196, Burkholderia xenovorans LB400, Delftia acidovorans SPH-1, Leptothrix cholodnii SP-6, Mycobacterium vanbaalenii PYR-1, Rubrivivax gelatinosus IL144, Thauera phenylacetica B4P and Pseudomonas putida SP902. These bacterial strains were mixed together, with or without P. putida. The community was grown for 2 weeks at 16°C, 90 rpm in a threefold-diluted Luria Bertani broth. Half of the broth was refreshed after a week with fresh medium. After 2 weeks, DNA was extracted and quantitative PCR analyses (qPCR) have been performed using specific primers targeting the 16S rRNA gene for each bacterial strain. Each of the primer pairs have been tested for specificity (see previous reporting periods) and only amplify their respective target DNA. qPCR measurements are in progress and will be presented in WP2.
Main achievements in respect to initial WP objectives
We successfully designed 2 synthetic communities and were able to analyse their composition and their functionality using new proteomic approaches. The diversity of the communities was calculated using the Shannon Wiener index. However, this index was not useful as only one bacterium dominated in the community. Thanks to recent results, we are now able to thoroughly describe the function of a community. Previous experiments made on diversity and invasion (see previous reports) will now be reproduced at a larger scale to annotate the already strong conclusions we obtained: One bacterium was always dominating the community and invasion depended on the invader strength.
Deviations from initial WP objectives
Initially, synthetic communities were cultivated in 200 µL wells of a microtiter plate, in order to improve statistics and data volume for the WP5. Using this approach, we encountered many contamination and evaporation problems. Therefore, we decided to grow synthetic communities in flasks. We subsequently increased the time scale of the analysis to 1 month. Renewal of the medium, which is the main contributor to contamination problems, has also been reduced to only once a week (for the river community). A novel flow-through system will be soon implemented in the lab. Growth of the community in biofilms will also be investigated as described in WP3.
Synthetic communities with more than 12 species were originally planned. However it was difficult in the laboratory because prices and time of Q-PCR analysis increased with the number of species.
At last, Horizontal Gene Transfer experiments were planned for the WP1. It was decided to start this work directly in WP3, i.e. on biofilms placed in a flow-through system (see WP3).
INT1: EAWAG
Objectives
To assist MRM researchers with the establishment of advanced FCM methods for bacterial analysis.
Scientific activities and results
EAWAG hosted UGent researchers in 2013, 2014 and 2016 and collaborated in these visits on several FCM-related projects. (a) We established operational protocols for high-throughput FCM. (b) We tested and demonstrated fully-automated real-time flow cytometry in conjunction with advanced FCM fingerprinting methods.
Current research activity with respect to microbial invasion in GAC filters is still on-going.
INT2: DTU
The comments below are for WP1 to 4 as DTU contributed to all in the same way.
Objectives
Support the analysis of the spatial structure of microbial communities
Scientific activities and results
- Supported the network on microbial ecology theory
- Development a conceptual framework for invasion in microbial communities
Main achievements in respect to initial WP objectives
The invasion framework was published in a high impact journal, where we provide rigorous descriptions of the invasion process and definitions of the constitutive elements as well as theoretical foundation to interpret (and possibly control) this process
Deviations from initial WP objectives
The research of the network focused less than planned on the role of spatial structure in microbial communities hence the decision of focusing on contributing to firming up the theory behind MRM
2.2. WP2. Microbial interactions in synthetic communities under selective stress.
2.2.1.Involved partners
All Belgian partners (UGent, UCL, KUL, UMons) were involved with WP2. In WP2 the synthetic ecosystems from WP1 were subjected to selective stress. P2 (UCL) was the WP leader.
2.2.2.Summary description of the objectives
The main objective of WP2 to assess the influence of environmental stressors and/or stimuli on the model microbial communities developed in WP1 in terms of resilience and robustness.
2.2.3.Summary of the scientific activities and results per partner
P1: UGent (KERMIT) – supporting role (to P3)
Objectives
Task 2.1: To investigate how the diversity of the endogenous community affects the invasion of pesticide degrading inoculants in sand filter ecosystems under selective conditions
Scientific activities and results
- Performed statistical analyses in order to determine the presence and significance of correlations between BAM-degradation performance of MSH1 (as measured by mineralization parameters) and the presence of (combinations of) sand filter isolates
- Performed statistical analyses in order to determine the presence and significance of correlations between BAM-degradation performance of MSH1 (as measured by mineralization parameters) and the survival of MSH1 after two competition phases
- Development of a regression model to predict pairwise mineralization parameters, based on individual mineralization parameters
Main achievements in respect to initial WP objectives
Task 2.1:
- The results of the statistical analyses were used to help determine how the diversity (richness) of the endogenous community affected the degradation of BAM by MSH1 in sand filter ecosystems under selective conditions.
- The predictive regression model was used to highlight combinations of sand filter isolates that produced unexpected mineralization behaviour, so that these might be further investigated via in vitro experiments
P2: UCL
Objectives
Task 2.2, Soil ecosystems:
Polycyclic aromatic hydrocarbons (PAHs) biodegradation using synthetic bacterial communities under selective pressure.
Task 2.3, Marine ecosystems:
Additional objective related to marine ecosystems: Dimethylsulfoniopropionate (DMSP) biotransformation using bacterial communities under specific relevant selective conditions
Additional task, Wastewater Ecosystems
Additional objective related to wastewater ecosystems: Treatment of synthetic wastewater at 4 °C using artificial, psychrophilic bacterial communities under selective pressure.
Scientific activities and results
Task 2.2, Soil ecosystems:
- Biodegradation kinetics of a mixture of PAHs (PHE, FLU, ANT) in the presence of heavy metals (Cu, Zn and Cd, ranging from 0.01 to 0.6 mM of each metal: Figure 2‑10) using combinatorial biodiversity experiments (full factorial design, from monocultures to tricultures).
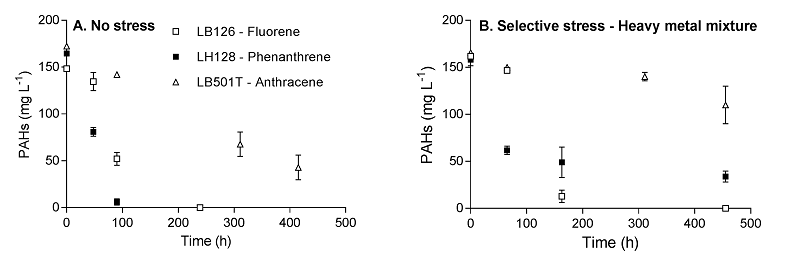
Figure 2‑10. Biodegradation kinetics of fluorene, phenanthrene and anthracene by pure cultures of strain LB126, LH128 and LB501T, respectively. Initial PAH concentrations were set to 150 mg L-1. A. Biodegradation in the absence of heavy metals (no stress). B. Biodegradation in the presence of heavy metals (a mixture of Cd, Cu and Zn, 0.8 mM each).
Task 2.3, Marine ecosystems:
- DMSP biodegradation in batch reactors and continuous mixed flow reactors (CMFRs) in the presence of oil spill dispersants (e.g., Dioctyl sodium sulfosuccinate (AOT), a Corexit component) and fluctuations of temperature or pH.
- Effects of environmental conditions on the routing of DMSP biodegradation pathways (demethylation pathway cleavage pathway, Figure 2‑11)
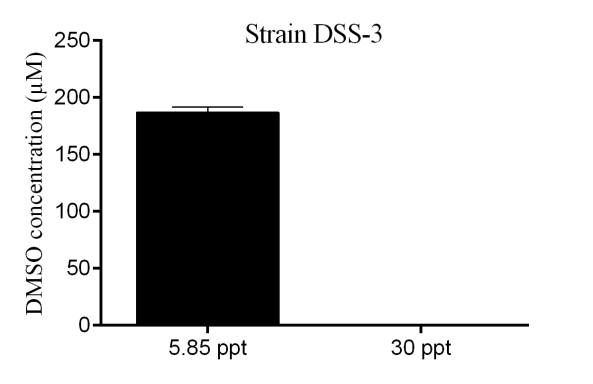
Figure 2‑11. Comparison of DMSO production (used as a proxy for DMSP cleavage to DMS) under different salinity levels (30 ppt and 5.85 ppt) for the strainDSS-3 grown on DMSP 2 mM after 6 days of incubation.
WP2, Additional task, Wastewater Ecosystems
- Kinetics of sCOD removal by fluctuating initial COD concentrations.
Main achievements in respect to initial WP objectives
WP2, Task 2.2, Soil ecosystems
Coupling of biodiversity, functionality and robustness (resistance and resilience) of PAH-degrading mixed cultures.
P3: KUL
Objectives
WP2, Task 2.1, Sand filter ecosystems
Determine the invasion of MSH1 in biofilms of synthetic communities in oligotrophic conditions and effect of Assimilable Organic Carbon (AOC) content and study the occurrence of HGT of catabolic genes for BAM degradation.
Scientific activities and results
WP2, Task 2.1, Sand filter ecosystems
- Invasive behavior of MSH1 was assessed under different levels of selectivity and different scenarios of ecological succession.
- Continuously fed flow cells were used to determine the invasive and colonizing behavior of MSH1 under oligotrophic conditions in biofilms in the absence and presence of a synthetic community of 13 SFIs. The sand filter community was either allowed to colonize the system before adding MSH1 or together with MSH1.
- When simultaneously colonizing a surface with the SFI, apparent competition with resident bacteria was observed as MSH1 formed separate colonies from SFI. When invading an existing SFI community, MSH1 showed cooperative behavior as became apparent from integration in the SFI biofilm and the increase of certain SFI in abundance after invasion.
- The availability of AOC on the invasive behavior was assessed. Higher AOC led in general to an increase in MSH1’s BAM degrading activity. Interestingly, at low AOC concentrations, the presence of the SFI community improved BAM degradation
- HGT in mono-species biofilms of MSH1 was studied under oligotrophic conditions and increasing selective conditions i.e. increasing BAM concentrations
- The occurrence of two subpopulations within the MSH1 population was observed. One subpopulation carries the plasmid for BAM degradation to DCBA (90-99%), while the second subpopulation carries the latter plasmid and the plasmid for DCBA mineralization (1-10%). HGT among these subpopulations of the plasmid for DCBA-mineralization was assessed in biofilms grown under oligotrophic conditions with varying levels of BAM and AOC. Increasing BAM concentrations led to an increase in the BAM mineralizing subpopulation. Lower BAM concentrations and the presence of AOC led to a decrease in this group. The dynamics in abundance of both subpopulations was not due to HGT or a loss of the plasmid for BAM mineralization, but due to a difference in growth rates of both populations.
Main achievements in respect to initial WP objectives
- Invasion of sand filter bacterial biofilms is affected by the stage of ecosystem development. When a endogenous community is already present, the invader (MSH1) was more successful and showed cooperative behavior with the resident bacteria. When added together, the invader (MSH1) was less successful and showed competitive behavior. The availability of additional organic carbon increased invasiveness of the sand filter community.
- HGT of the two plasmids responsible for full BAM mineralization does not occur within the MSH1 population. Currently, HGT to resident sand filter bacteria wasn’t assessed but seems to be unlikely under oligotrophic conditions
P4: UMons
Objectives
Task 1.3, Marine Ecosystems
It is generally recognized that stress and/or stimuli shape microbial communities. The marine sediments that were selected to serve as a model in the present study are polluted by numerous metals (Gillan et al. 2015) and some of the strains selected in WP1 present various metal-resistance systems. After consideration, we decided to use zinc as a stressor.
The UMons objectives within WP2 were therefore to analyze the effect of zinc 0.5 mM on the diversity and the functionality of the community prepared in WP1.
Scientific activities and results
Task 1.3, Marine Ecosystems
At first, the Minimum Inhibitory Concentration (MIC) for zinc was determined for each strain. Most of the strains were resistant to 0.5 mM zinc after 48h of growth in SSM8 (16°C, 90 rpm) (e.g., P. putida), while others were not able to grow even at 0.5 mM Zinc in SSM8 (e.g., Shewanella baltica). Cupriavidus metallidurans, known to be resistant to various metal stresses (MIC zinc 5 mM in rich medium, data not shown) was surprisingly sensitive to zinc (MIC = 1.5 mM after more than 50 hours). This may be an effect of the SSM8 growth medium in which Cupriavidus has difficulties to grow.
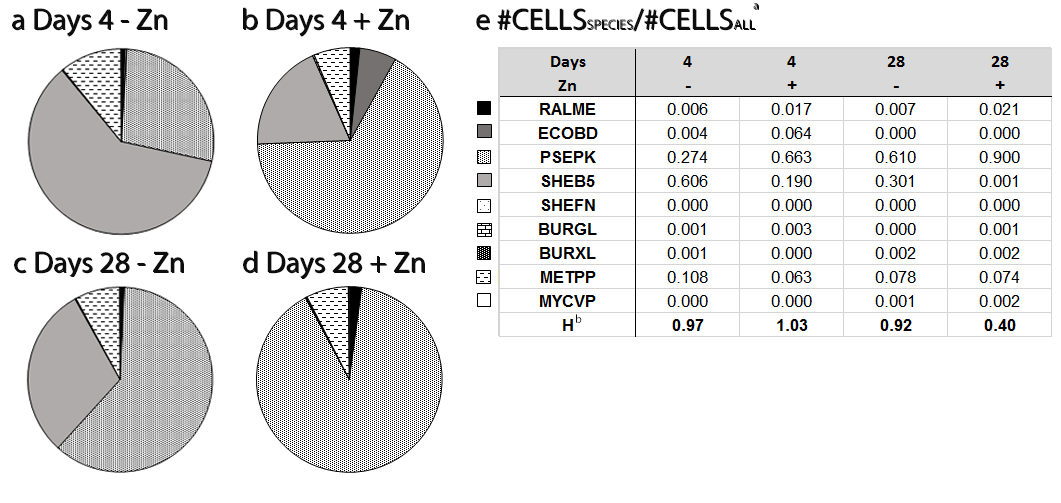
Figure 2‑12. a-d : Pie charts representing the abundances of theoretical CELLS for each species inside the community after 4 days (a, b) or 28 days (c, d) in SSM8 (a, c) or SSM8 with zinc 0.5 mM (b, d) at 16°C, 90 rpm. 16S DNA composition was assessed by Q-PCR and transformed into theoretical number of cells. Legend and data table are given in (e). RALME, ECOBD, PSEPK, SHEB5, SHEFN, BURGL, BURXL, METPP and MYCVP stand for Cupriavidus metallidurans, Escherichia coli, Pseudomonas putida, Shewanella baltica, Shewanella frigidimarina, Burkholderia glumae, Burkholderia xenovorans, Methylibium petroleiphilum and Mycobacterium vanbaalenii respectively. a #CELLSSPECIES/CELLSALL has been calculated from Q-PCR 16S DNA composition, 16S DNA/cell per species and resulting total number of cells. b Shannon-Wiener diversity index.
According to the literature, a zinc concentration of 0.5 mM in a minimal medium is sufficient to activate zinc resistance in Cupriavidus as well as in Pseudomonas. It was decided to conduct an experiment with a complete synthetic community and a zinc concentration of 0.5 mM (sensitive bacteria such as S. baltica, might survive the stress with the help of the other strains).
A 2-weeks old community composed of the 9 strains used in WP1 was grown in triplicates in SSM8 (16°C, 90 rpm) before being divided in two parts: one half was maintained in SSM8 medium (control), the other in SSM8 + zinc 0.5 mM. After 4 and 28 days of exposure to Zn, quantitative PCR (qPCR) was used to determine the relative abundance of the strains in the community (Figure 2‑12).
As expected, the presence of Zn significantly affected the structure of the synthetic community over time. S. baltica totally disappeared after 28 days of exposure to Zn. This was expected according to its weak resistance to Zn (MIC= 0.5 mM). Particularly interesting were the abundances of Escherichia coli and Cupriavidus metallidurans. According to qPCR, the proportion of E. coli was higher after 4 days of exposure when zinc was present. However, after 28 days, E. coli disappeared in all conditions (Zn-amended and controls). Proportions of C. metallidurans on the other hand were higher in the presence of Zn (qPCR, Figure 2‑12) after 4 and 28 days. Because of their low proportions in the communities (up to 2% at the best for C. metallidurans) and their specific pattern according to the conditions, these 2 species have been targeted first during the SWATH-MS experiments.
Hydrophilic proteins have been prepared from the same samples after 4 and 28 days. After trypsin digestion, the proteins have been analyzed by DIA (Mass spectrometry using SWATH acquisition, see WP1). Thanks to this method, it was possible to quantify the abundance of proteins from specific species inside the whole proteome of the community. This is the first time that the State-of-the-Art SWATH acquisition method (Gillet et al., 2012) was used in a synthetic community. A technical short communication will be submitted in the coming month (Beraud et al., in preparation). Another manuscript is expected in the coming year after reproduction of the results. This manuscript should gather the results of WP1 and WP2, and conclusions about influence of Zn 0.5 mM on community function. First results are presented here.
Two quantitative parameters will be used to study the influence of Zn 0.5 mM on the community:
- Total Area: Protein abundance computed using total transition area and normalized using total area obtained for each sample. Here, “Total Area” also refers to the sum of all proteins (Total Area of a sample).
- Log2(Ratio): The base 2 logarithm of the ratio between two conditions for a specific protein or a specific condition. Three biological replicates are made for each condition, and p-values are computed using a Student T-test.
The Total Area for a sample gives an idea of the abundance of each protein in the community. When summing the total area of the proteins according to species, we can have an overall idea of the functional presence of the bacteria inside the community (Figure 2‑13).
So far we only analyzed 3 bacteria: E. coli (ECOBD), C. metallidurans (RALME) and P. putida (PSEPK). When comparing the Total Area obtained for each species, results are quite similar compared to what have been observed by qPCR: PSEPK is by far more represented than ECOBD and RALME. Furthermore, ECOBD is less present after 28 days than after 4 days.
From these analysis, 585 proteins from Pseudomonas putida as well as 85 proteins from C. metallidurans and 76 proteins from E. coli were identified (745 proteins in total). This was the first time during the project that we could see the proteins from rare species using mass spectrometry.
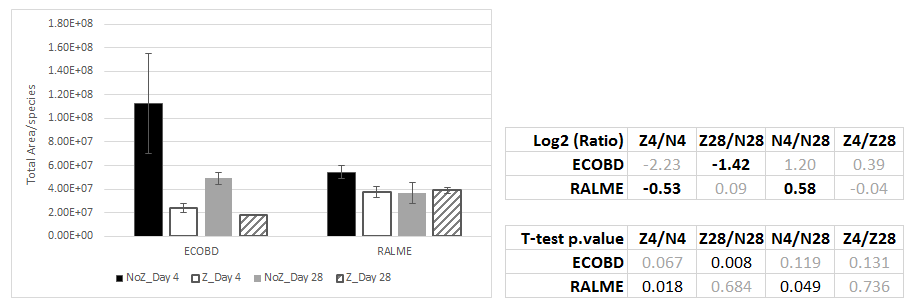
Figure 2‑13. Total Area of proteins detected using SWATH-MS per species. 4 assays have been tested: Communities have been cultured in triplicates at 16°C, 90 rpm in SSM8 (“N4”, “N28”) or SSM8 + zn 0.5 mM (“N28”, “Z28”) for 4- (“Z4”, “N4”) or 28- days (“Z28”,”N28”). Hydrophilic proteins have been prepared, then digested using trypsin. Resulted peptides have been analysed using SWATH- MS and a Spectral library composed of 3 species (Pseudomonas putida, Escherichia coli (“ECOBD”) and Cupriavidus metallidurans (“RALME”). Total Area has been computed after normalization and resulting Log2 (Ratio between the conditions) are given as well as p-value from Student T-test.
Glycolysis proteins, ribosomal proteins, amino acids biosynthesis and GroEL protein from E. coli were disappearing with time in controls (4 to 8-fold less abundant) as well as at Day_4 in the presence of zinc. This confirms what was already obvious with the qPCR (Figure 2‑12) and Total Area (Figure 2‑13) : E. coli disappears and become inactive between Day_4 and Day_28. It cannot sustain the Zn stress either. The presence of some proteins involved in oxidative stress repair such as the Thioredoxin or the Peptide methionine sulfoxide reductase increase in the presence of zinc at both time points. Oxidative stress proteins are known to be expressed after a metal stress (Gillan, 2016).
For C. metallidurans, the proteomic profile in the community confirms its low abundance. After 28 days, ribosomal proteins suggest a higher activity in the controls. But Lipid biosynthesis, and a couple of induced transport systems suggest that inside the community, Cupriavidus is still active and interacting with its environment. When C. metallidurans is analysed in single bacterial cultures using Data-dependent Acquisition Mass Spectrometry (DDA-MS), some Czc, Cop and Cup proteins (involved in metal resistance) are upregulated in the Zn-contaminated SSM8 medium. Particularly, CzcB increased 5-fold (data not shown). None of these metal-resistance proteins were detected by SWATH-MS in the synthetic community. But other proteins, such as glutathione transporter (Q1LNI5), and phosphate/phosphonate metabolism systems (Q1LLB2, Q1LJ09), or oxidative stress response proteins (Q1LEU4) have already been proposed to react to the presence of metal stressors .
For the invasion experiments in microtiter plates in the presence of Zn, the invasion pattern of a community was assessed by qPCR using Pseudomonas putida, Cupriavidus metallidurans and Escherichia coli as invaders. No clear effects of Zn for C. metallidurans invasion was found after 5-days. Conversely, Zn at 0.5, 1.0 and 1.5 mM increased the capacity of P. putida and E. coli to invade a community composed of 5 bacteria. Although the qPCR data needs to be confirmed by proteomic approaches, it is certain that Zn triggers a stress-response in P. putida and E. coli. These responses may then increase their ability to invade the community. In other words, invasion would be facilitated by stress.
At last, for the river sediment synthetic community (WP1) MIC for various metals of each strain have been described at 16°C, 90 rpm. To mimic the stress encountered by this community in natural environment, Zn at a concentration of 0.5 mM final or Pb at a concentration of 1.5 mM final have been added to the medium. In parallel, 3 assays have been kept metal-free (LB).
After 14 days, 1.5 to 5 mL of the cultures have been pelleted and DNA was extracted. Similar to previous experiments (made on the sea sediments community), qPCR primers have been designed to specifically detect the 16S rRNA gene of each of the strain. Quality of the primers, sensibility and sensitivity of the amplification have been assessed.
So far, only qPCR for Pseudomonas putida, Thauera phenylacetica, Azoarcus communis and Delftia acidovorans were performed. The preliminary graphs can be found in Figure 2‑14.
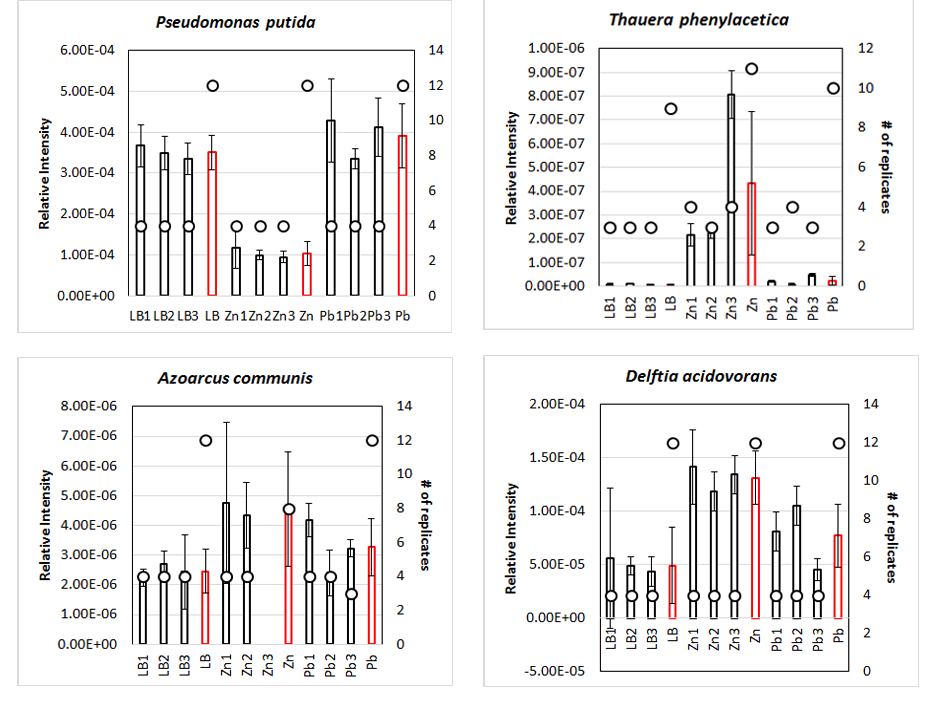
Figure 2‑14. qPCR analysis of river sediment communities. Average Relative Intensity of the 16S DNA of 4 species (Pseudomonas putida, Thauera phenylacetica, Azoarcus communis and Delftia acidovorans) is represented using bar graph on left Y- axis. Communities have been made in 3-fold diluted LB (biological replicates “LB1”, ”LB2”, “LB3”), added with zn 0.5 mM (biological replicates “Zn1”, ”Zn2”, “Zn3”) or Pb 1.5 mM (biological replicates “Pb1”, ”Pb2”, “Pb3”). Averages and standard deviation for each condition is given (red bar, “LB”, “Zn”, “Pb”). Number of biological and/or technical replicates used to compute previous averages are represented on the right Y- Axis (circle).
Results need to be confirmed and statistical analyses must be completed. But regarding these first results, it is clear that Pseudomonas putida and Delftia acidovorans are both quite abundant in the community compared to Azoarcus communis and Thauera phenylactetica. Furthermore, P. putida seems to be less abundant with zinc, which is consistent with the sensitivity of the strain to zinc. On the contrary, T. phenylacetica and D. acidovorans, both resistant to concentration > 1.5 mM Zn were more abundant in the presence of zinc. Communities elaborated without P. putida as well as functional analysis using SWATH-MS analysis will be used to further explore these communities
Main achievements in respect to initial WP objectives
We have analysed the final diversity and functionality of a marine community in the presence of a zinc stress. Thanks to qPCR experiments, shifts in the composition of the community, due to metal addition, have been visualized. In almost every conditions, P. putida was dominating the community. So far, functional analysis seems to confirm the positive role of Zn on C. metallidurans. It is more abundant with Zn than without. Further proteomic analyses are needed to conclude : is it because other strains such as P. putida and S. baltica are disappearing or because C. metallidurans is favored?
Furthermore, the river sediment community, composed of a new set of bacteria present the same pattern. Q-PCR analysis and proteomics experiments from the coming month should give clues about the link between diversity and functionality.
INT1: EAWAG
Objectives
To advise and assist MRM researchers with biodegradation experimental set-ups in low nutrient environments.
Scientific activities and results
EAWAG is hosting and training a KUL researcher in the use of FCM and working in AOC free environments (on-going at time of writing). Current research activity with respect to microbial invasion in GAC filters is still on-going.
2.3. WP3. Synthetic communities in a sterile environmental matrix
2.3.1.Involved partners
All Belgian partners (UGent, UCL, KUL, UMons) were involved with WP2. P3 (KUL) was the WP leader.
2.3.2.Summary description of the objectives.
Ecological hypotheses will be evaluated using the model microbial communities from WP1, in their natural, but sterile habitats.
2.3.3.Summary of the scientific activities and results per partner.
P1: UGent (KERMIT) - Supporting role (to P3).
Objectives
Task 3.1: To investigate how the diversity of the endogenous community affects the invasion of pesticide degrading inoculants in sand filter ecosystems in a sterile environmental matrix
Scientific activities and results
- Development of a combinatorial experimental design based on covering arrays, in order to test a sampling of richness combinations of sand filter isolates (from one to thirteen), since testing all possible combinations was unfeasible
- Performed statistical analyses in order to determine the presence and significance of correlations between BAM-degradation performance of MSH1 (as measured by mineralization parameters) and the presence of (combinations of) sand filter isolates
- Performed statistical analyses in order to determine the presence and significance of correlations between BAM-degradation performance of MSH1 (as measured by mineralization parameters) and the survival of MSH1 after two competition phases
- Performed statistical analyses in order to determine the statistical significance of SFI identity effects at richness level one, i.e. whether the presence of a particular SFI resulted in statistically significantly different mineralization parameters
- Performed statistical analyses in order to determine the statistical significance of SFI identity effects at richness level two, i.e. whether the presence of a particular SFI in a pair combination resulted in statistically significantly different mineralization parameters
Main achievements in respect to initial WP objectives
The results of the statistical analyses are being used to help determine how the diversity (richness) of the endogenous community affected the degradation of BAM by MSH1 in sand filter ecosystems in a sterile environmental matrix
P2: UCL
Objectives
Task 3.2, Soil ecosystems:
Polycyclic aromatic hydrocarbons (PAHs) biodegradation by artificial bacterial communities within a sterile, soil background matrix.
Task 3.3, Marine ecosystems:
Additional objective related to marine ecosystems: Dimethylsulfoniopropionate (DMSP) biotransformation by bacterial communities within sterile, marine water.
Additional Task, Wastewater ecosystems
Additional objective related to wastewater ecosystems: Treatment of sterile, real wastewater at 4 °C using artificial, psychrophilic bacterial communities.
Scientific activities and results
Test of Sterilization protocol: autoclaving soil three times (121 °C, 45 min) over 3 consecutive days, at 24 h intervals. Intermediate incubation periods of 24 h at room temperature will be carried out to kill sporulating microorganisms. To inhibit fungal growth, cycloheximide (actidione, 50 µg mL-1) will also be added in sterile systems. Other experiments and tasks within this WP are still on-going.
Main achievements in respect to initial WP objectives
At UCL the work on WP3 is still in development hence no achievements with respect to the initial WP objectives can be reported yet.
Deviations from initial WP objectives
At UCL the work on WP3 is still in development hence no deviations with respect to the initial WP objectives can be reported.
P3: KUL
Objectives
Task 3.1, Sand filter ecosystems
Determine invasion of MSH1 in a sterile sand filter matrix with and without a synthetic community
Scientific activities and results
Invasive behavior of MSH1 in sterile lab scale sand filters: Sand filters continuously fed with sterile water in the absence or presence of a synthetic community showed that no apparent effect of the community was observed in the initial invasive phase.
Main achievements in respect to initial WP objectives
At KUL the work on WP3 is still in development hence no achievements with respect to the initial WP objectives can be reported yet.
Deviations from initial WP objectives
At KUL the work on WP3 is still in development hence no deviations with respect to the initial WP objectives can be reported.
P4: UMons
Objectives
Task 1.3, Marine Ecosystems
The aim of UMons within WP3 was to use fluorescent mobile DNA to follow genetic transfers inside a synthetic community. For this, bacterial strains have been genetically modified and tools have been designed (sterilised environmental matrices and glassware).
Scientific activities and results
Task 1.3, Marine Ecosystems
Several studies report on the importance of mineral particles for bacterial communities. Mineral particles, such as sand are a surface for bacterial attachment and biofilm formation. It was reported that biofilms are important for formation and maintenance of a bacterial community as well as for resistance to various stresses (including invasion). Some of the bacteria used in the community (like Escherichia coli) were shown to survive in marine environment only due to the presence of sediments. Furthermore, natural seawater contains numerous carbon sources that may influence the composition of the synthetic community.
We designed an efficient protocol to sterilize environmental water and sediments, i.e. to remove living organisms while keeping the physico-chemical composition of the medium unaffected. Three different sterilization procedures have been tested so far on fresh river water harvested the day before. Unfortunately, these results were unsuccessful: autoclaving was the most efficient sterilisation method but failed to inactivate all bacterial spores. In addition, autoclaving affected the chemical composition of the water (its pH). No further experiments have been conducted so far on this point. In parallel, glass beads (diameter of 104 µm) have been selected to elaborate an artificial sediment on which bacterial strain will be able to elaborate a biofilm. First biofilm test experiments have been started (data not shown).
Two mobile genetic elements (a plasmid and a genomic island), featuring Pb resistance systems, will be tagged with fluorescent proteins in order to be able to track their movements in the synthetic communities. This part of the work is in progress (Valentine Cyriaque, PhD student, in Prof. S. Sørensen laboratory). For that, the strain Pseudomonas putida KT2440 with the fluorescent plasmid pKJK5-pA10403-gfpmut3-Km (Bahl et al., 2009) is used. This strain contains the fluorescent protein GFP that is repressed in the host strain (P. putida). The GFP on the plasmid is only expressed when the plasmid is transferred to a receiver strain. Plasmid transfer is thus easily detected via fluorescent microscopy. A low-copy number version of the plasmid, pLENT, containing pbrUTRABCD, involved in lead resistance in C. metallidurans was constructed using MuA-transposition in E. coli. The construct will be transferred into P. putida.
Mobile genetic elements known as ICE (Integrative Conjugative Elements) are among the most abundant conjugative elements in the prokaryotic world and may subsequently be very important in shaping communities (Guglielmini et al., 2011). As a consequence, we decided to also tag an ICE in Delftia acidovorans SPH-1with GFP. The pbr system must also be inserted in the strain. The HGT experiments (tagged plasmid and ICE) will be done in simulated river sediment communities designed earlier (by a Master student, Marta Brodzik; see WP1, WP2). The tagged P. putida donor will also be used on the synthetic marine community (see WP1 and 2).
Finally, in a manuscript soon to be published (Cyriaque et al., in preparation) the plasmidome of a natural sediment bacterial community in freshwater, held in microcosms for 6 months, was examined. Some of the microcosms were treated with Zn, others were left untreated. The 16S rRNA composition of the community is currently being analyzed by Illumina sequencing.
Main achievements in respect to initial WP objectives
On the date of this ex-post evaluation UMons only started working on WP3 a few months ago. So far, the glassware has been selected and the genetic manipulations (tagged plasmid with GFP) are almost finished. We expect first results in the coming year. If successful, the experiments with tagged ICE elements will be the first in synthetic communities.
Deviations from initial WP objectives
So far, the sterilization process for the natural matrices has been unsuccessful. We are now using synthetic medium to mimic natural sediments. Experiments made so far have shown no big differences between river water and MilliQ water, as long as carbon sources are added.
2.4. WP4. Validation in autochthonous communities
2.4.1. Involved partners
All Belgian partners (UGent, UCL, KUL, UMons) were involved with WP4. P4 (UMons) was the WP leader.
2.4.2. Summary description of the objectives
In WP4 the results obtained in the previous work packages were validated for natural ecosystems.
2.4.3. Summary of the scientific activities and results per partner
P2: UCL
Objectives
Task 4.2, Soil ecosystems
Polycyclic aromatic hydrocarbons (PAHs) biodegradation by artificial bacterial communities within a non-sterile, soil background matrix.
Task 4.3, Marine ecosystems
Additional objective related to marine ecosystems: Dimethylsulfoniopropionate (DMSP) biotransformation by bacterial communities within non-sterile, marine water.
Additional Task, Wastewater ecosystems
Additional objective related to wastewater ecosystems: Treatment of real wastewater (non-sterile) at 4 °C using artificial, psychrophilic bacterial communities.
Scientific activities and results
The work on WP4 at UCL is still preliminary, no results can be reported yet.
P3: KUL
Objectives
Task 4.1, Sand filter ecosystems
Determine invasion of MSH1 in pilot scale sand filters in DWTP
Scientific activities and results
Task 4.1, Sand filter ecosystems
Invasive behavior of MSH1 in sterile and non-sterile lab scale sand filters fed with non-sterile groundwater
Invasion of MSH1 in sand filters continuously fed with groundwater containing the residing groundwater bacteria was assessed for sterile sand filter columns and for sand filter columns previously primed with a sand filter community coming from DWTPs. The continuous supply of groundwater bacteria led to reduction in BAM removal both in the absence or presence of a synthetic community after a period of time. No apparent effect of the sand filter community was observed in the initial invasive phase. However, the continuous supply of bacteria probably led to a loss of MSH1 from the sand filters and as a consequence also BAM-degrading activity was lost. Important to note was that the priming of the sand filter with extracted communities from sand filter in DWTPs extended the period before loss of BAM-degrading activity occurred. As such the occurrence of other species can have a positive effect on survival of the invader at a later stage.
Invasive behavior of MSH1 in pilot scale sand filters continuously fed with BAM-contaminated groundwater of a DWTP
The bioaugmentation of MSH1 in pilot scale sand filters was assessed under different strategies of cell delivery (suspended or carrier embedded cells). Species richness and evenness of the system and how this is impacted by bioaugmentation will be determined.
Main achievements in respect to initial WP objectives
At KUL the work on WP4 is still in development hence no achievements with respect to the initial WP objectives can be reported yet.
Deviations from initial WP objectives
At KUL the work on WP4 is still in development hence no deviations with respect to the initial WP objectives can be reported.
P4: UMons
Objectives
Task 4.3, Marine ecosystems
The aim of WP4 is to reach the higher complexity and use the results obtained during the first three WP.
Scientific activities and results
Task 4.3, Marine ecosystems
Sediments have been harvested from two sites along the same river. The first site, MetalEurop, is located close to a former foundry closed since 2003 and still contaminated with various metals. The second site, Férin, situated upstream, presents the same physicochemical properties than MetalEurop except for the metal contamination. In a manuscript (Cyriaque et al., in prep) we have compared the plasmidome of the two communities and have performed microcosm experiments.
For that, 50 g of sediments from Férin have been harvested and transferred into the microcosms. Sediments were kept at 15°C, 70 rpm and overlaying water was changed periodically (autoclaved water from Férin), every week. After a week of equilibration, gradual additions of Zn (to reach a final concentration of 2870 mg/kg; "Zn samples") or Cd, Cu, Pb & Zn (to reach 36.8 mg/kg, 86.3 mg/kg, 902.2 mg/kg and 2870 mg/kg; “M” or “M+” samples) have been performed over an 8 weeks period. The final theoretical concentration of each metals correspond to the concentration published for sediments in MetalEurop. Bioavailable metal concentrations have been checked. For each condition, sediments have been made in triplicate, including the controls (no metals added). Every 2 months, viable counts as well as Denaturing Gradient Gel Electrophoresis (DGGE) analysis have been performed (using a fragment of the 16S rRNA gene) (Figure 2‑16)
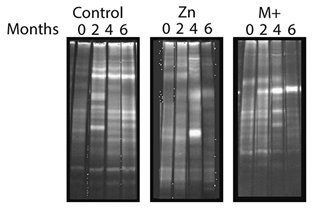
Figure 2‑16. DGGE profile evolution of 16S DNA in each microcosm conditions. Natural sediments have been transferred in microcosm and kept for 6 months at 15°C, 70 rpm. After 1 week, metal have been gradually added in the microcosms over a period of 8 weeks. Samples were: “Control” (no addition); “M+” (cadmium (36.8 mg/kg theoretical concentration), copper (86.3 mg/kg theoretical concentration), lead (902.2 mg/kg theoretical concentration) and zinc (2870 mg/kg theoretical concentration)); “Zn” (zinc 2870 mg/kg theoretical concentration). DGGE analysis of the resulting sediments communities have been done every 2 months.
Only 1 band was clearly visible in the DGGE profile of M+ samples after 6 months. Consistent with the results from WP2 on the synthetic sea water community (section 2.2.3), metal addition reduces the evenness of the community of natural river sediments. This band has been sequenced and was shown to represent Sphingomonadaceae, i.e. α-Proteobacteria known to degrade aromatic compounds.
The plasmidome was analysed using shotgun metagenomics after 6 months and compared to natural sediments (“T0”). Plasmid host taxonomic composition are reported in Figure 4.2.
Microcosm incubation had an influence on plasmid host taxonomic composition (Figure 2‑17). Numerically, E. coli plasmids dominate in all microcosms despite the fact that the bacterium was not far detected abundant with DGGE analysis. This suggests that the microcosm experiment promoted the transfer of E. coli plasmids and related functions without particularly favoring the abundance of the bacterium in itself. E. coli could be an important vector of horizontal gene transfer in freshwater.
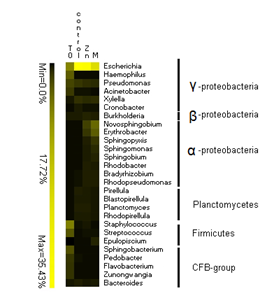
Figure 2‑17. Heatmap of plasmid host genus diversity from the mobilome of Férin (T0) and each microcosm condition (control, Zn, M) including the 10 most abundant genera for all of them. The colour intensity is related to the abundance (%) of each genus (annotation was performed using M5NR database)
Another interesting result is that metal additions have led to an increase in the abundance of α-Proteobacterial plasmids. This was consistent with observations made in Férin and MetalEurop by metagenomics (Cyriaque et al., in preparation). α-Proteobacteria were also more abundant in contaminated microcosms M+ after 6 months (Figure 2‑17). In this particular case, plasmid abundance increases with host abundance.
Functional annotation of the plasmidome revealed that metal resistance systems are significantly more abundant in microcosms contaminated with metals. Levels of the cobalt-zinc-cadmium resistance system (Czc) increased 2 times in the Metaleurop plasmidome. qPCR assays have been performed to follow czcA but problems occurred because metallic contaminants inhibited the PCR.
The next step will be to use the fluorescent strains (developed in WP3) in similar microcosms and follow the transfer of the plasmid or ICE element in natural sediments. As transfer does not mean protein expression, improvements of the SWATH-MS approach to analyse specific proteins from the sediments will be tested to see if the selected resistance mechanisms are expressed in the new hosts.
Main achievements in respect to initial WP objectives
The WP4 only started recently. So far we could produce results similar to those observed in the synthetic community regarding the diversity of the community. Selective extraction of the plasmidome proved efficient. A manuscript will be published soon (Cyriaque et al., in preparation) and new experiments will start as soon as the tools developed during WP3 are available.
Deviations from initial WP objectives
The qPCR approach was not effective for the metal-contaminated sediments placed in microcosms. However, the Illumina sequencing technology was more appropriate and finally less time consuming and less expensive.
2.5. WP5. The modelling framework (in parallel with WP1 through 4)
2.5.1.Involved partners
The UGent partner (KERMIT, Bernard De Baets) and international partner 2 (DTU, Barth Smets) as well as international partner 3 (KU, Søren Sørensen) were involved in WP5. P1 (UGent, KERMIT) was the WP leader.
2.5.2.Summary description of the objectives
In WP5 in in silico models were developed in parallel to W1 to 4 to increase (mechanistic) insights into (synthetic) ecosystems which can be tuned with parameters obtained from the in vitro work within the other WPs.
2.5.3.Summary of the scientific activities and results per partner.
P1: UGent - KERMIT
Objectives
Task 5.1: Simplified synthetic ecosystems
In this task we aimed to construct a spatiotemporal modelling framework to mechanistically explain hypotheses regarding community biodiversity and functionality (particularly in the face of competition between species).
Task 5.2: Synthetic ecosystems with selective conditions
In this task we aimed to introduce variable environmental conditions to the modelling framework, as such expressing the dependence of crucial microbial processes on such changing conditions using functions rather than parameter values.
Task 5.3: Synthetic systems and autochthonous communities on an environmental matrix
In this task we aimed to parameterize and validate our models, to permit a quantitative assessment of the essential microbial ecosystem characteristics, such as the species abundance and their spatial distribution.
Scientific activities and results
For this WP, there is some overlap between our scientific activities and results, since for example developing an IBM is an activity, with the result being the IBM itself.
A spatially explicit individual-based modelling framework and extension modules (e.g. additional species, variable community evenness, different competition structures, fixed/non-fixed competition outcomes, interaction with changing environment, resource-dependent demographic processes) were developed. Subsequently, In silico experiments using above IBMs, conducted using High Performance Computing (HPC) infrastructure at UGent were performed and followed by a Sensitivity analysis of IBM.
Deviations from initial WP objectives
Our scientific activities have proceeded according to the initial WP objectives.
INT2: DTU
Objectives
Contribute to the modelling efforts
Scientific activities and results
Review and compare modelling framework for microbial co-metabolism
Main achievements in respect to initial WP objectives
This comparison was published in a high impact journal
Deviations from initial WP objectives
The research of the network focused less than planned on the role of spatial structure in microbial communities
INT3: KU
Objectives
Development of domain specific language tools for bioinformatics analysis.
Scientific activities and results
Development of bioinformatics tools, sequencing, travel for collaborative meetings and organization for the consortium and IT. Specifically for the development of workflow pipelines for multiple step data processing in, for example, multiple FASTA entries and analysis was adapted for parallelization, validated in practical work and published online. Validation was performed during the completion of the Ph.D. study performed by P.N. Holmsgaard and scientific work detailed in publications below was based on the pipelines described here.
BioDSL (pronounced Biodiesel) is a Domain Specific Language for creating bioinformatics analysis workflows. A workflow may consist of several pipelines and each pipeline consists of a series of steps such as reading in data from a file, processing the data in some way, and writing data to a new file. BioDSL is built on the same principles as Biopieces, where data records are passed through multiple commands each with a specific task. The idea is that a command will process the data record if this contains the relevant attributes that the command can process. E.g. if a data record contains a sequence, then the command reverse_seq will reverse that sequence.
2.6. Annual and other meetings
Annually, a meeting was coordinated by the UGent partner with each of the different partners hosting the meeting. Always presentations were given by each of the partners to exchange research and get input and experimental design recommendations. Additionally an IAP business meeting was organized.
Apart from annual meetings the individual partners organized meetings as need, some of which are listed below:
- UMons has a punctual meeting with WP5 partners (KERMIT) in order to set up joint experiments.
- Multiple meetings between KUL and UMons partners to discuss (meta)proteomic approaches
- Multiple meetings between KUL, CMET and KERMIT partners to coordinate KERMIT/CMET/KUL collaboration on WPs 1, 2 and 3.
- KERMIT: Multiple meetings with CMET partners (Ramiro Vilchez Vargas, Frederiek-Maarten Kerckhof, Nico Boon) to plan experiments for WP5
- In the beginning of the project, several workshops were organized.
- Regular tele-meetings between EAWAG and KUL regarding working with AOC and BAM catabolic pathway elucidation